| |
  Page 21 of 29 Page 21 of 29   |
| Author |
Message |
VK4AYQ
Guru

Joined: 02/12/2009
Location: AustraliaPosts: 2539 |
| Posted: 12:58am 01 Jan 2015 |
 Copy link to clipboard Copy link to clipboard |
 Print this post |
|
Hi All
Progress report on the battery bank.
Over the holiday I got inspired and reassembled battery bank number 2 the one I have been re hydrating for the last couple of months, it had dried out something fierce as I ended up putting nearly 40 Ltr's of water in it, it has been a slow process as it takes time for the plate matrix to re saturate, topping up morning and night with 10 cc at a time.

For those that may be looking at doing this exercise this is how I check each cell to avoid overfilling.

The dip stick is a bamboo skewer pinched fro the kitchen, after a few days the acid starts to break down the end and it then resembles a small short hair paint brush.
Above the plate matrix is a plastic cover and my aim is to fill to that level, I am not sure what the level was originally so this may be a slight overfill but I think better to have a little too much rather than not enough electrolyte as in time it will slowly evaporate anyway.
When the water level is below the plastic cover and you dip the cell the stick will be damp but not wet so a wipe on the top of the battery shows no beading moisture.

It is a bit hard to see in the picture but if you look carefully you can see a stain line across the cover heading toward the terminal.

In contrast to the previous mark in this photo you can see a bead of electrolyte across the cover. When the electrolyte is above the plastic it is quite a definite indication, all you have to do is to make sure you wipe the moisture of the dip stick before testing the next cell.
It is a slow process but the batteries have been on charge with the desulphator all the time so it has given them time to recover as well as re hydrate.

Bank 2 batteries back in the rack with desulphator fitted across the 24 volt set on each level it is reconnected to the solar and during the day sits on 30 volts to assist the process ff desulphation.
I am now doing bank 1 which is in a lot better condition so it is going quicker, a couple of weeks should get it re hydrated.
Sorry if I am boring you with to much detail, but it may be of use to you some day.
All the best
Bob
Foolin Around |
| |
gww1
Regular Member

Joined: 14/06/2013
Location: United StatesPosts: 63 |
| Posted: 07:11am 01 Jan 2015 |
 Copy link to clipboard Copy link to clipboard |
 Print this post |
|
I am not bored.
gww |
| |
dwyer
Guru

Joined: 19/09/2005
Location: AustraliaPosts: 574 |
| Posted: 03:14pm 01 Jan 2015 |
 Copy link to clipboard Copy link to clipboard |
 Print this post |
|
I am not bored but enjoyed Bob'passion and i promise visit Bob last year due too much other commitments
dwyer  |
| |
VK4AYQ
Guru

Joined: 02/12/2009
Location: AustraliaPosts: 2539 |
| Posted: 04:23pm 01 Jan 2015 |
 Copy link to clipboard Copy link to clipboard |
 Print this post |
|
Hi Dwyer
I look forward to seeing you this year, coffee or tea is always on.
All the best
Bob
Foolin Around |
| |
Georgen
Guru

Joined: 13/09/2011
Location: AustraliaPosts: 462 |
| Posted: 11:04am 02 Jan 2015 |
 Copy link to clipboard Copy link to clipboard |
 Print this post |
|
I am regular reader of this thread too.
Hope to venture into 'desulphator' ownership one day, but I am so scared to build one that I probably will buy.
George |
| |
VK4AYQ
Guru

Joined: 02/12/2009
Location: AustraliaPosts: 2539 |
| Posted: 01:22pm 02 Jan 2015 |
 Copy link to clipboard Copy link to clipboard |
 Print this post |
|
Hi George
I know how you feel about the new IC stuff and I am a bit of a dinosaur myself but I think the design I have seen are simple enough for me to have a go at it, as I want to put a series of desulphators in three banks of batteries totaling 18 desulphator units the cost of commercial units would be around $1200 so that's a real incentive to have a go at making my own. Also want to put one on each vehicle and the reactor and on the 12 volt lighting set up on the house.
At the moment I am running Infinity commercial units but as the Pacific Peso is nose diving, and the cost of more of those becomes prohibitive, around $80 each landed here, while they are good units and I have used mine for over three years and only managed to blow one and let out the magic smoke, so good units, and I have desulphated many batteries with them including my battery banks,cost has reared it's ugly head, pension only goes so far.
When I get some working I will try to get a thread going to help others to do the same, with all comments and suggestions by our hobby community welcome.
The spare money is better spent on an upgraded Inverter and some more panels for a bit more capacity and also the battery fund that will be needed when my batteries finally give up the ghost.
All the best
Bob
Foolin Around |
| |
Georgen
Guru

Joined: 13/09/2011
Location: AustraliaPosts: 462 |
| Posted: 11:19pm 02 Jan 2015 |
 Copy link to clipboard Copy link to clipboard |
 Print this post |
|
Hi Bob
Would like to have a go, but definitely will see if I can make my baby steps in electronics construction once you put out some spoon feeding material on the web.
In a meantime I built crude lead acid batteries understanding:
1. Deep cycle batteries can last up to 12 maybe 20 years if not discharged much.
2. Batteries with thicker lead plates the better
3. I got impression that desulphator can be connected to battery on day one for the life of battery.
Does it make any sense, or I got it totally wrong?
George |
| |
VK4AYQ
Guru

Joined: 02/12/2009
Location: AustraliaPosts: 2539 |
| Posted: 02:09am 03 Jan 2015 |
 Copy link to clipboard Copy link to clipboard |
 Print this post |
|
Hi George
Your questions
1. Deep cycle batteries can last up to 12 maybe 20 years if not discharged much.
The batteries I have been fooling with are 15 years old and have a design life for commercial replacement of 10 years, they are an extremely well built battery but being sealed construction the electrolyte slowly evaporates over the years, the lead plates in them are 5 mm thick and it is my opinion with recharge earlier than I have done it they would have gone for 20 years assuming a discharge of no more than 60%. Some of the old glass cell I serviced in the dark ages where 30 years old and still going, but like all things if abused the life span is cut down drastically. I see that several Lead Acid deep cycle battery makers are now saying 20 year life span for their product and I believe this achievable with proper management. NIFE cells are good for 50 years but double the price and still require some management.
2. Batteries with thicker lead plates the better
The more lead in the plates the longer they will last, but with mismanagement they will also have a shortened lifespan, the weight of a battery is a good indicator of its quality, a car battery that is light will last about 3 years and with better management and a desulphator fitter can go to five or six years. A battery needs to be worked to last longer, in the case of a car type battery a 30% discharge is about right, for deep cycle you can go to 60% discharge without to much damage, as long as it is not left in that state, needs to be charged right away, as do all lead acid batteries. Cycling within these limits will prolong the useful life of any battery be it a cheepy or a deep cycle.
3. I got impression that desulphator can be connected to battery on day one for the life of battery.
There is a number of opinions on this one, so I can only speak from my experience.
In the dark ages I used a desulphator to revive large 32 volt batteries that had been sulphated by years of abuse by farmers not doing enough charging to keep the batteries active, it was a slow process and would bring a battery back from the dead if still mechanically sound, from 10% capacity to better than 80% in most cases, but in those days there was no commercial desulphators available so it was a one shot thing, in the end I made the point of going around my customers twice a year and giving the batteries a burst with the desulphator. Then the rural power by SWER lines was rolled out, and that was the end of that.
Now I use desulphators on my batteries all the time and do believe it keeps them in better condition, my battery bank is now 15 years old, it was a standby emergency lighting set up in a government building when I got them they where partly sulphated from sitting in the scrap yard, after 3 months they came to nearly full capacity and have maintained that until the last episode when they finally dried out due to age as the spec sheet gives a spec on liquid loss per year in service.
This is largely my fault as I have been ill and hospitalized a number of times in the last year and didn't give enough notice to what was happening, I was finally alerted to their distress when the sulphate on the outside of the cell pack expanded to the point of splitting the cases on a few batteries, they where still working on the moisture held in the matrix so it wasn't immediately obvious. I have had to discard 4 batteries that have gone to far, and have four more still debatable but the rest are back to over 80% which makes them usable.
My opinion from experience not theory.
I believe that if a desulphator is fitted from day one and the equalization charge is reduced to float levels there would be a much lower loss of fluid from the battery and the constant desulphation of the plates result in a lower loss of active material from the plates thereby extending their life considerably.
I am going to fit a 12 volt desulphator across every 12 volt group in the battery bank when I get it all back together, at the moment I am using 24 volt desulphator across each level in the bank and while that is better than nothing I think the 12 volt solution would be better.
Summery
It is better to keep a battery on good condition than fix it when you stuff it up, and with less loss of plate grunge from boiling the sulphate off as in a high equalization voltage and current, there is less grunge build up in the bottom of the case to short out the bottom of the plates to create a higher than normal self discharge rate of in the extreme case a shorted cell.
All the best
Bob
Foolin Around |
| |
Georgen
Guru

Joined: 13/09/2011
Location: AustraliaPosts: 462 |
| Posted: 07:32pm 03 Jan 2015 |
 Copy link to clipboard Copy link to clipboard |
 Print this post |
|
Bob
Appreciate your help.
Hope you can type fast, as you did mighty job with all the explaining.
Looking at stationary battery bank, I get impression that flooded battery is not that bad option.
Alternatively sealed battery with tiny hole drilled to syringe in some water as reversal of slow evaporation, might be as good approach too.
With industrial batteries price might be fairly high, so sourcing and reviving second hand one might be my preferred option.
I have little area out of sight under the balcony.
But from time to time I have to trap mice there and occasional rat.
Is there a danger of rat or mice having a go at plastic battery box?
Would it be necessary to put battery into some kind of metal protection, or there is little chance for vermin getting attracted to lead acid battery?
George |
| |
VK4AYQ
Guru

Joined: 02/12/2009
Location: AustraliaPosts: 2539 |
| Posted: 08:03pm 03 Jan 2015 |
 Copy link to clipboard Copy link to clipboard |
 Print this post |
|
Hi George
I have never had trouble with mice or rats on my batteries a bit of possum poop occasionally that's all. I don't think they like the taste or smell of a battery and a bit of ratsak sprinkled around would help if you don't have a resident snake.
My experiment with the drilled holes in sealed batteries seems to be working OK, when I Finnish with the re hydration I will block the holes with short bits of Oring material to keep the grot out and make the pressure valves in the caps work properly.
A good option is ex forklift batteries a lot of the service people pull them out after a specific period (company maintenance schedule) and sell on the good ones at reasonable prices, I have been quoted $650 for a 750 AH battery 24 volt, with a bit of TLC and a permanent desulphator it should do a few more years even if it is down a little on maximum capacity, forklift batteries are very tough batteries and if you can pick a good one it will last for years in our application. They generally come in a metal container but are very heavy so you would need some form of material handling device unless you dismantle and move a cell at a time.
There are lots of good options around but expensive and I am not so sure that the bottom end of the deep cycle market are as good as made out to be, same goes for gel cell as there is a dehydration problem with them too, Downwind was re hydrating some he has but I haven't heard if it was a success or not.
All the best
Bob
Foolin Around |
| |
VK4AYQ
Guru

Joined: 02/12/2009
Location: AustraliaPosts: 2539 |
| Posted: 08:32pm 04 Jan 2015 |
 Copy link to clipboard Copy link to clipboard |
 Print this post |
|
Progress report
I checked the batteries from bank 1 today and found they had all equalized at 7 volts per battery so am now going to run a slight cycling load on them for a few days using a grid feed inverter and solar drive with charger on the side to monitor any additional current draw.

This is the two lower shelves of the bank, I had one failed battery in this lot and one suspect.

This is the top shelf batteries fron Number 1 bank several had suffered heat damage from the fire I had but have now equalized after re hydration.
A total of 24 ltr's have gone into these to get the hydrated again, it would have been only a short time before they went the way of bank 2 as it is I have lost one and another one is weak.
They have been sitting on 30 volts for a week now and all batteries are within .1 volts of each other when I first connected them they varied from 6.5 to 8 volts indicating the high voltage ones where sulphated, now they have equalized a week or so of light cycling load should bring them back good enough to put back in the rack and do some work again. The working in the top 10 to 20 percent of capacity while the desulphator is running does a lot of good for the restoration of overall capacity.
On bank 2 I have put equalizer straps at middle of the banks so I can install 12 volt desulphators when i get around to making them, nearly all the required parts have arrived today so I will have to get my finger out.
All the best
Bob
Foolin Around |
| |
isaiah

Guru

Joined: 25/12/2009
Location: United StatesPosts: 303 |
| Posted: 10:05pm 04 Jan 2015 |
 Copy link to clipboard Copy link to clipboard |
 Print this post |
|
Bob and all
Bub built some desulfaters 12 volt like you guys are working on and they have worked good as long as you can keep the moisture out of them.
We need to make some more up.
I got another lawn mower battery that had been let set for 3=4 yrs. it is up to 8 volts at rest now.
We put it inside tonight as its supposed to get around "0" f for several days.
We put one of the home made desulfaters on and will see what it is in the mourning.
Running the desulfater self powered
This one has been funny some times it would peg the charger out then the next day charge like it should. It wants to go slow.
Keep up the good job and show pictures of the progress
Isaiah
URL=http://www.motherearthnews.com/Renewable-Energy/1973-11- 01/The-Plowboy-Interview.aspx>The Plowboy Interview[/URL> |
| |
VK4AYQ
Guru

Joined: 02/12/2009
Location: AustraliaPosts: 2539 |
| Posted: 01:01pm 05 Jan 2015 |
 Copy link to clipboard Copy link to clipboard |
 Print this post |
|
Hi Isaiah
Moisture and corrosive atmosphere around batteries is a bit of a problem on electronics, I came across some fully sealed project boxes for $2 that could solve the problem, a good lashing of CRC and at least it is serviceable, one of the commercial ones I have is fully potted in epoxy, good for reliability but not for repair.
I find that once you can get the battery to take a charge it is better to use a low amps charge 1 or two amps in conjunction with the desulphator as it does less damage to the plates while converting the sulfides, what you are describing could be a piece of plate material shorting out the cell then breaks down and goes back to a normal charge, I found this happened if the battery is turned over as it gets the grunge from the bottom of the cell across the plates and can go from a short to a high resistance that causes self discharge in the cell, try tapping the battery lightly with a hammer while watching the amps charge, that will tell if anything is moving around and causing problems. One of the batteries I tried the ALUM thing on had a lot of shed plate material wash out when I rinsed it, black flakes of grunge and lead sulfide.
All the best
Bob
Foolin Around |
| |
M Del
Senior Member

Joined: 09/04/2012
Location: AustraliaPosts: 155 |
| Posted: 03:14pm 05 Jan 2015 |
 Copy link to clipboard Copy link to clipboard |
 Print this post |
|
Hi Guys
Rats and mice stay away from batteries, something to do with the smell and slight burning sensation. They do however like the taste of brake fluid, I have replaced many brake and clutch reservoirs over the years as they chew through them to get the moisture.
Mark |
| |
VK4AYQ
Guru

Joined: 02/12/2009
Location: AustraliaPosts: 2539 |
| Posted: 04:46pm 05 Jan 2015 |
 Copy link to clipboard Copy link to clipboard |
 Print this post |
|
Hi Mark
They could be frustrated alcoholics, and with not many brain cells to kill it may not make much difference until they die and then nobody cares. HE HE
I had something chew a hole in a container of brake fluid in my shed so you have explained that,
All the best
Bob
Foolin Around |
| |
isaiah

Guru

Joined: 25/12/2009
Location: United StatesPosts: 303 |
| Posted: 08:09pm 05 Jan 2015 |
 Copy link to clipboard Copy link to clipboard |
 Print this post |
|
Bob and All,
We just had a experience with the stator out of a old fan and found that
McMaster-Carr ( http://www.mcmaster.com ) has a spray insulating varnish ($6.10 or so they have 3 grades) that is used by a lot of the fan restorers.there is some Red spray varnish on ebay and etc but they say it dosent work as good as the stuff from McMaster-Carr
I have a stator that had a bad buzz in it and several coats of the spray varnish and we heated it to 170 deg F to cure it and no more buzz. maybe a coat or two would be good on the desulphators and should be able to replace parts if needed!
This picture shows the stator in front of the infrared heater

Isaiah
URL=http://www.motherearthnews.com/Renewable-Energy/1973-11- 01/The-Plowboy-Interview.aspx>The Plowboy Interview[/URL> |
| |
VK4AYQ
Guru

Joined: 02/12/2009
Location: AustraliaPosts: 2539 |
| Posted: 05:15pm 06 Jan 2015 |
 Copy link to clipboard Copy link to clipboard |
 Print this post |
|
Hi Isaiah
That's a good idea I have some two pac esterpol that would do the trick. Cool set so as not to cook the electronics.
All the best
Bob
Foolin Around |
| |
VK4AYQ
Guru

Joined: 02/12/2009
Location: AustraliaPosts: 2539 |
| Posted: 09:03pm 06 Jan 2015 |
 Copy link to clipboard Copy link to clipboard |
 Print this post |
|
Hi All
I had a nice visit today from Rustyron and Shirley (XYL) we had a chance to compare ideas on desulphators and various circuits, two heads is defiantly better than one, roy has been a busy boy and constructed a few different circuits and showed me a circuit for one that desulphate Canberra, well maybe, I will be opting for a more conservative approach as I believe that the most beneficial process is to run a desulphator all the time rather than waiting till there is sulphate to be got rid of, to maintain batteries in tip top condition is very important.
While it is good to be able to reconstitute an old battery as a challenge, it is more important for us to keep our new / good banks in top condition, they are a big investment that needs to be protected.
All the best
Bob
Foolin Around |
| |
VK4AYQ
Guru

Joined: 02/12/2009
Location: AustraliaPosts: 2539 |
| Posted: 11:08am 07 Jan 2015 |
 Copy link to clipboard Copy link to clipboard |
 Print this post |
|
A bit of theory to help understand what happens to our batteries when we discharge them, from an old battery book from 1922
WHAT TAKES PLACE DURING DISCHARGE
Considered chemically, the discharge of a storage battery consists of the changing of the spongy lead and lead peroxide into lead sulphate, and the abstraction of the acid from the electrolyte. Considered electrically, the changes are more complex, and require further investigation. The voltage, internal resistance, rate of discharge, capacity, and other features must be considered, and the effects of changes in one upon the others must be studied. This proceeding is simplified considerably if we consider each point separately. The abstraction. of the acid from the electrolyte gives us a method of determining the condition of charge or discharge in the battery, and must also be studied.
Voltage Changes During Discharge. At the end of a charge, and before opening the charging circuit, the voltage of each cell is about 2.5 to 2.7 volts. As soon as the charging circuit is opened, the cell voltage drops rapidly to about 2.1 volts, within three or four minutes. This is due to the formation of a thin layer of lead sulphate on the surface of the negative plate and between the lead peroxide and the metal of the positive plate. Fig. 21 shows how the voltage changes during the last eight minutes of charge, and how it drops rapidly as soon as the charging circuit is opened. The final value of the voltage after the charging circuit is opened is about 2.15-2.18 volts. This is more fully explained in Chapter 6. If a current is drawn from the battery at the instant the charge is stopped, this drop is more rapid. At the beginning of the discharge the voltage has already had a rapid drop from the final voltage on charge, due to the formation of sulphate as explained above. When a current is being drawn from the battery, the sudden drop is due to the internal resistance of the cell, the formation of more sulphate, and the abstracting of the acid from the electrolyte which fills the pores of the plate. The density of this acid is high just before the discharge is begun. It is diluted rapidly at first, but a balanced condition is reached between the density of the acid in the plates and in the main body of the electrolyte, the acid supply in the plates being maintained at a lowered density by fresh acid flowing into them from the main body of electrolyte. After the initial drop, the voltage decreases more slowly, the rate of decrease depending on the amount of current drawn from the battery. The entire process is shown in Fig. 22.
Lead sulphate is being formed on the surfaces, and in the body of the plates. This sulphate has a higher resistance than the lead or lead peroxide, and the internal resistance of the cell rises, and contributes to the drop in voltage. As this sulphate forms in the body of the plates, the acid is used up. At first this acid is easily replaced from the main body of the electrolyte by diffusion. The acid in the main body of the electrolyte is at first comparatively strong, or concentrated, causing a fresh supply of acid to flow into the plates as fast as it is used up in the plates. This results in the acid in the electrolyte growing weaker, and this, in turn, leads to a constant decrease in the rate at which the fresh acid flows, or diffuses into the plates. Furthermore, the sulphate, which is more bulky than the lead or lead peroxide fills the pores in the plate, making it more and more difficult for acid to reach the interior of the plate. This increases the rate at which the voltage drops.
The sulphate has another effect. It forms a cover over the active material which has not been acted upon, and makes it practically useless, since the acid is almost unable to penetrate the coating of sulphate. We thus have quantities of active material which are entirely enclosed in sulphate, thereby cutting down the amount of energy which can be taken from the battery. Thus the formation of sulphate throughout each plate and the abstraction of acid from the electrolyte cause the voltage to drop at a constantly increasing rate.
Theoretically, the discharge may be continued until the voltage drops to zero, but practically, the discharge should be stopped when the voltage of each cell has dropped to 1.7 (on low discharge rates). If the discharge is carried on beyond this point much of the spongy lead and lead peroxide have either been changed into lead sulphate, or have been covered up by the sulphate so effectively that they are almost useless. Plates in this condition require a very long charge in order to remove all the sulphate.
The limiting value of 1.7 volts per cell applies to a continuous discharge at a moderate rate. At a very high current flowing for only a very short time, it is not only' safe, but advisable to allow a battery to discharge to a lower voltage, the increased drop being due to the rapid dilution of the acid in the plates.
The cell voltage will rise somewhat every time the discharge is stopped. This is due to the diffusion of the acid from the main body of electrolyte into the plates, resulting in an increased concentration in the plates. If the discharge has been continuous, especially if at a high rate, this rise in voltage will bring the cell up to its normal voltage very quickly on account of the more rapid diffusion of acid which will then take place.
The voltage does not depend upon the area of the plate surface but upon the nature of the active materials and the electrolyte. Hence, although the plates of a cell are gradually being covered with sulphate, the voltage, measured when no current is flowing, will fall slowly and not in proportion to the amount of energy taken out of the cell. It is not until the plates are pretty thoroughly covered with sulphate, thus making it difficult for the acid to reach the active material, that the voltage begins to drop rapidly. This is shown clearly in Fig. 22, which shows that the cell voltage has dropped only a very small amount when the cell is 50% discharged. With current flowing through the cell, however, the increased internal resistance causes a marked drop in the voltage. Open circuit voltage is not useful, therefore to determine how much energy has been taken from the battery.
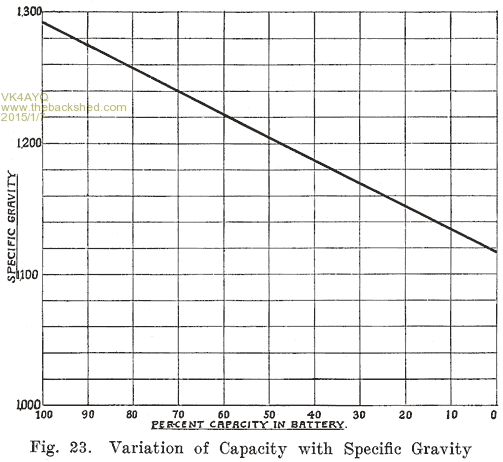
Acid Density. The electrolyte of a lead storage battery is a mixture of chemically pure sulphuric acid, and chemically pure water, the acid forming about 30 per cent of the volume of electrolyte when the battery is fully charged. The pure acid has a "specific gravity" of 1.835, that is, it is 1.835 times as heavy as an equal volume of water. The mixture of acid and water has a specific gravity of about 1.300. As the cell discharges, acid is abstracted from the electrolyte, and the weight of the latter must therefore grow less, since there will be less acid in it. The change in the weight, or specific gravity of the electrolyte is the best means of determining the state of discharge of a cell, provided that the cell has been used properly. In order that the value of the specific gravity may be used as an indication of the amount of energy in a battery, the history of the battery must be known. Suppose, for instance, that in refilling the battery to replace the water lost by the natural evaporation which occurs in the use of a battery, acid, or a mixture of acid and water has 'been used. This will result in the specific gravity being too high, and the amount of energy in the battery will be less than that indicated by the specific gravity. Again, if pure water is used to replace electrolyte which has been spilled, the specific gravity will be lower than it should be. In a battery which has been discharged to such an extent that much of the active material has been covered by a layer of tough sulphate, or if a considerable amount of sulphate and active material has been loosened from the plates and has dropped to the bottom of the cells, it will be impossible to bring the specific gravity of the electrolyte up to 1.300, even though a long charge is given. There. must, therefore, be a reasonable degree of certainty that a battery has been properly handled if the specific gravity readings are to be taken as a true indication of the condition of a battery. Where a battery does not give satisfactory service even though the specific gravity readings are satisfactory, the latter are not reliable as indicating the amount of charge in the battery.
As long as a discharge current is flowing from the battery, the acid within the plates is used up and becomes very much diluted. Diffusion between the surrounding electrolyte and the acid in the plates keeps up the supply needed in the plates in order to, carry on the chemical changes. When the discharge is first begun, the diffusion of acid into the plates takes place rapidly because there is little sulphate clogging the pores in the active material, and because there is a greater difference between the concentration of acid in the electrolyte and in the plates than will exist as the discharge progresses. As the sulphate begins to form and fill up the pores of the plates, and as more and more acid is abstracted from the electrolyte, diffusion takes place more slowly.
If a battery is allowed to stand idle for a short time after a partial discharge, the specific gravity of the electrolyte will decrease because some, of the acid in the electrolyte will gradually flow into the pores of the plates to replace the acid used up while the battery was discharging. Theoretically the discharge can be continued -until all the acid has been used up, and the electrolyte is composed of pure water. Experience has shown, however, that the discharge of the battery should not be continued after the specific gravity of the electrolyte has fallen to 1.150. As far as the electrolyte is concerned, the discharge may be carried farther with 'safety. The plates determine the point at which the discharge should be stopped. When the specific gravity has dropped from 1.300 to 1.150, so much sulphate has been formed that it fills the pores in the active material on the plates. Fig. 23 shows the change in the density of the acid during discharge.
Changes at the Negative Plate. Chemically, the action at the negative plate consists only of the formation of lead sulphate from the spongy lead. The lead sulphate is only slightly soluble in the electrolyte and is precipitated as soon as it is formed, leaving hydrogen ions, which then go to the lead peroxide plate to form water with oxygen ions released at the peroxide plate. The sulphate forms more quickly on the surface of the plate than in the inner portions because there is a constant supply of acid available at the surface, whereas the formation of sulphate in the interior of the plate requires that acid diffuse into the pores of the active materials to replace that already used up in the formation of sulphate. In the negative plate, however, the sulphate tends to form more uniformly throughout the mass of the lead, because the spongy lead is more porous than the lead peroxide, and because the acid is not diluted by the formation of water as in the positive plate.
Changes at the, Positive Plate. In a fully charged positive plate we have lead peroxide as the active material. This is composed of lead and oxygen. From this fact it is plainly evident that during discharge there is a greater chemical activity at this plate than at the negative plate, since we must find something to combine with the oxygen in order that the lead may form. lead sulphate with the acid. In an ideal cell, therefore, the material which undergoes the greater change should be more porous than the material which does not involve as great a chemical reaction. In reality, however, the peroxide is not as porous as the spongy lead, and does not hold together as well.
The final products of the discharge of a positive plate are lead sulphate and water. The lead peroxide must first be reduced to lead, which then combines with the sulphate from the acid to form lead sulphate, while the oxygen from the peroxide combines with the hydrogen of the acid to form water. There is, therefore, a greater activity at this plate than at the lead plate, and the formation of the water dilutes the acid in and around the plate so that the tendency is for the chemical actions to be retarded.
The sulphate which forms on discharge causes the active material to bulge out because it occupies more space than the peroxide. This causes the lead peroxide at the surface to begin falling, to the bottom of the jar in fine dust-like particles, since the peroxide here holds together very poorly.
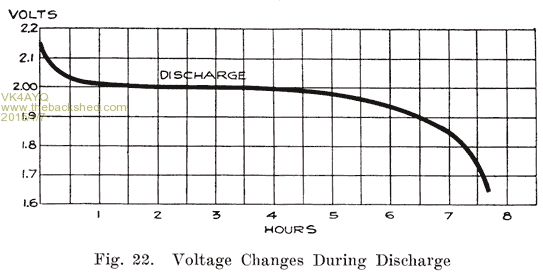
From this graph you can see that the efficiency of a battery chemically speaking drops after 50% discharge this is caused by the excess sulphation of the plates as they supply electro chemical energy to an external load.
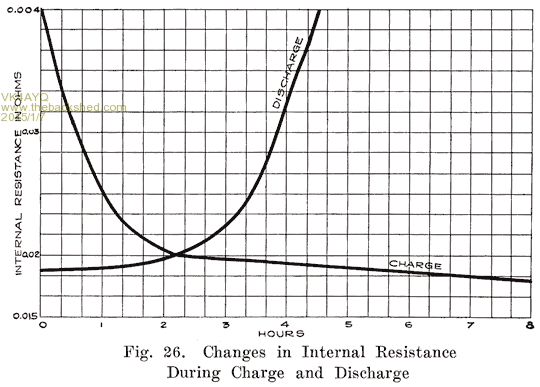
This is an interesting one as it shows the changes in internal resistance of a battery during discharge and recharge.
All the best
Bob
Edited by VK4AYQ 2015-01-08
Foolin Around |
| |
rustyrod

Senior Member

Joined: 08/11/2014
Location: AustraliaPosts: 121 |
| Posted: 06:19pm 07 Jan 2015 |
 Copy link to clipboard Copy link to clipboard |
 Print this post |
|
From Bob's text [Theoretically the discharge can be continued -until all the acid has been used up, and the electrolyte is composed of pure water.]
Thank you Bob that certainly clears it up for me and helps answer my own question in an earlier post about the batteries I used on my 32 volt.
I had asked “where did the acid go?”
I believe I now have found the answer.
Copied from internet ---(SULPHURIC ACID
Sulphuric acid in its pure form is a colourless viscous liquid commonly called oil of vitriol
The chemical formula for sulphuric acid is H2SO4 and it is commercially available as a 96-98% solution or 18M H2SO4.
Concentrated sulphuric acid is covalent in nature as the acid is unionised. The atoms in the molecule are covalently bonded to one another. In this form sulphuric acid can be transported in stainless steel tankers.
If concentrated sulphuric acid is added to water the reaction is extremely exothermic due to formation of hydrogen ions.
H2SO4(l) ==> H+(aq) + HSO4-(aq) First ionization
HSO4-(aq) ==> H+(aq) + SO42-(aq) Second ionization
Sulphuric acid is made from elemental sulphur or from its compounds by converting them to sulphur dioxide.
S + O2 ==> SO2
Sulphur + oxygen ==> Sulphur dioxide
SO2 + O2 ==> SO3
Sulphur dioxide + oxygen ==> Sulphur trioxide
SO3 + H2SO4 ==> H2S2O7
Sulphur trioxide + sulphuric acid ==> Oleum
H2S2O7 + H2O ==> 2H2SO4
Olem + water ==> Sulphuric acid
The direct addition of water to sulphur trioxide is too violent. That is why the sulphur trioxide is dissolved in sulphuric acid to form oleum. The oleum is then added to water to produce sulphuric acid).
I thought y’all would be SO exited to know all that and all those big words????
What is the relevance?
Well my friendly farmer has a few (lots) batteries under some trees, and those that have been there 20 years are empty/dry/dusty/spiders inside.
Those that have been there 4/5 years are almost empty.
Last years discards are full.
So I say the H20 has simply EVAPORATED.
Further examples
looking at my batteries picture, you will see one has the vent cap broken---- it is dry.
those with vents with red caps in -----are nearly full.
Those with vent caps and no red caps and blocked up -----are nearly full
Those with vent caps and no red caps -----are low.
The one with the broken terminal also has no gasket on the vent and no red cap, so full evaporation has emptied the cell.
More example
Sulfuric acid formula H2So4
Subtract the H2o and we have Lead (11) sulphate chemical formula of PbSo4
Clear as acid, Eh what?
Sulfuric acid (H2So4) minus Water (H2o) and add lead (Pb) and some oxide (O) and we have lead sulfate (PbSo4)
Or to put it simply -----a empty flat sulfated battery.
I should be mowing, but the grass will still be there another day, it will die back in the winter anyway, there is nothing worth watching on TV and it is much cooler inside with Google and the computer.
So Bob and all of us, it was not a lack of maintenance, over charging or ab/use that dried out the batteries after all.
You may all use this story when explaining to the finance department why you need a BIGGER new battery bank.
Of course you all welcome to correct me.
But it does sound “Plausible”
(I think I will go have a nap, all that Googling has made my finger tired)
rustyrod
Always Thinking |
| |
| |
  Page 21 of 29 Page 21 of 29   |

